Introduction
An ocular micrometer is a glass disk that fits in a microscope eyepiece that has a ruled scale, which is used to measure the size of magnified objects. The physical length of the marks on the scale depends on the degree of magnification. To put it simpy, an ocular micrometer used to measure the size of object and the stage micrometer is used to calibrate the ocular micrometer. This is to ensure the accurate measurement with ocular.
Materials
Microscope fitted with an ocular micrometer
Slide micrometer
Stained preparation of yeast
Method Used
1. Place the stage micrometer on the stage.
2. Using the lowest power objective, focus the microscope until the image on the stage micrometer is observed superimposed on the eyepiece scale.
3. Determine how many divisions of the eyepiece scale correspond top a definite number of divisions
on the stage scale.
4. Calculate the measurement of an eyepiece division in micrometer (μm).
5. Calculate and record for future reference, the diameter of the field for each objective.
6. Determine the average dimensions (in μm) of a sample of yeast cells.
Results
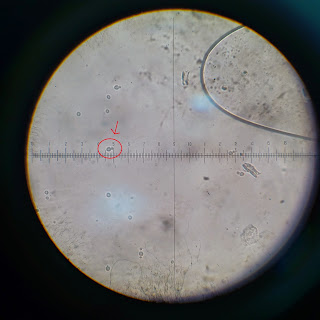
40 magnification image seen with ocular lens
100 magnification image seen with ocular lens
Average dimension of yeast:
10x objective lens - 1 mm = 9.5 ocular unit
40x objective lens - 0.1mm = 4 ocular unit
the length of the yeast microorganism
4 ocular unit = 0.1 mm
0.5 ocular unit = 0.0125 mm
the width of the yeast microorganism
4 ocular unit = 0.1 mm
0.3 ocular unit = 7.5x10^-3 mm
Discussion
-handle the yeast culture by using the aseptic technique to prepare the slide to be observed under ocular lens.-Calibrate the ocular lens to get the measurement.
10x objective lens - 1 mm = 9.5 ocular unit
40x objective lens - 0.1mm = 4 ocular unit
-to read the width and the length,rotate the objective len.
read the micrometer carefully to avoid parallex error.
2.2 Neubauer Chamber
Introduction
The hemocytometer (or haemocytometer or counting chamber) is a specimen slide which is used to determine the concentration of cells in a liquid sample. It is frequently used to determine the concentration of blood cells (hence the name “hemo-“) but also the concentration of sperm cells in a sample. The cover glass, which is placed on the sample, does not simply float on the liquid, but is held in place at a specified height (usually 0.1mm). Additionally, a grid is etched into the glass of the hemocytometer. This grid, an arrangement of squares of different sizes, allows for an easy counting of cells. This way it is possible to determine the number of cells in a specified volume.
Materials
Serial dilutions of yeast
Neubauer chamber and coverslip
70% ethanol
Sterile Pasteur pipette
Method Used
1. Using a sterile Pasteur pipette, add a drop of diluted yeast culture (use 10-3 or 10-4
2. Allow about one minute for the cells to settle dilution) to the space between the coverslip and the counting chamber.
3. Count the cells in the four corner and centre squares. For a reasonably accurate count, you should have more than 30 cells per area.
4. Clean the Neubauer and coverslip with 70% ethanol
Counting
1. The chamber contains many grids, producing nine (9) major large squares
2. For calculation purposes, only the middle large square is used.
3. The middle large square has a size of 1 mm x 1 mm and a depth of 0.1 mm
4. Inside the middle large squares, there are 20 smaller squares, each with the size of 0.2 mm x 0.2 mm
5. Randomly choose 12 out of 20 small squares and calculate the number of yeast cells in the squares
6. Average the number of cells per square.
Results
Image of yeast on hemocytometer (40x)
total cells of 12 small boxes = 237 cells
average for 1 small box = 237/12
=19.75 cells
total cells for 400 small boxes = 19.75 x 400
= 7900
volume of one small box = 1 mm x 1 mm x 0.1 mm
= 0.1 mm3
1 mm3 = 0.001 cm3
0.1 mm3 = 0.0001 cm3
1 cm3 = 1 ml
0.0001 cm3 = 0.0001 ml
concentration of cells in 400 small boxes = 7900cell /0.0001 ml
= 79000000 cell/ml
total cells of 12 small boxes = 237 cells
average for 1 small box = 237/12
=19.75 cells
total cells for 400 small boxes = 19.75 x 400
= 7900
volume of one small box = 1 mm x 1 mm x 0.1 mm
= 0.1 mm3
1 mm3 = 0.001 cm3
0.1 mm3 = 0.0001 cm3
1 cm3 = 1 ml
0.0001 cm3 = 0.0001 ml
concentration of cells in 400 small boxes = 7900cell /0.0001 ml
= 79000000 cell/ml
Discussion
-The yeast culture is put in the hemocytometer by using aseptic techniques. The
coverslip must be on the hemocytometer to gives a very precise volume in the space
delimited by the grid and the coverslip.It is being done carefully to avoid the air bubbles
in the counting chamber.
-The hemocytometer is thicker than a regular slide. We careful to not crash the objective
lens into the hemocytometer at the time of focusing.
-Choose 12 boxes out of 400 boxes and calculate the number of cells in each boxes.
Then get the average number of the cell to calculate the concentraion of the cell.
Conclusion
Finally, we would like to conclude that our group members have learned the correct techniques to measure the size of yeast by using an ocular micrometer with accurate measurement. We can also count the yeast by using a hemocytometer so we can determine the number of yeast in a specified volume.
Reference
- http://en.wikipedia.org/wiki/Ocular_micrometer
- http://www.wartburg.edu/biology/vlg/scale.html
- http://en.wikipedia.org/wiki/Hemocytometer
- http://www.microbehunter.com/the-hemocytometer-counting-chamber/